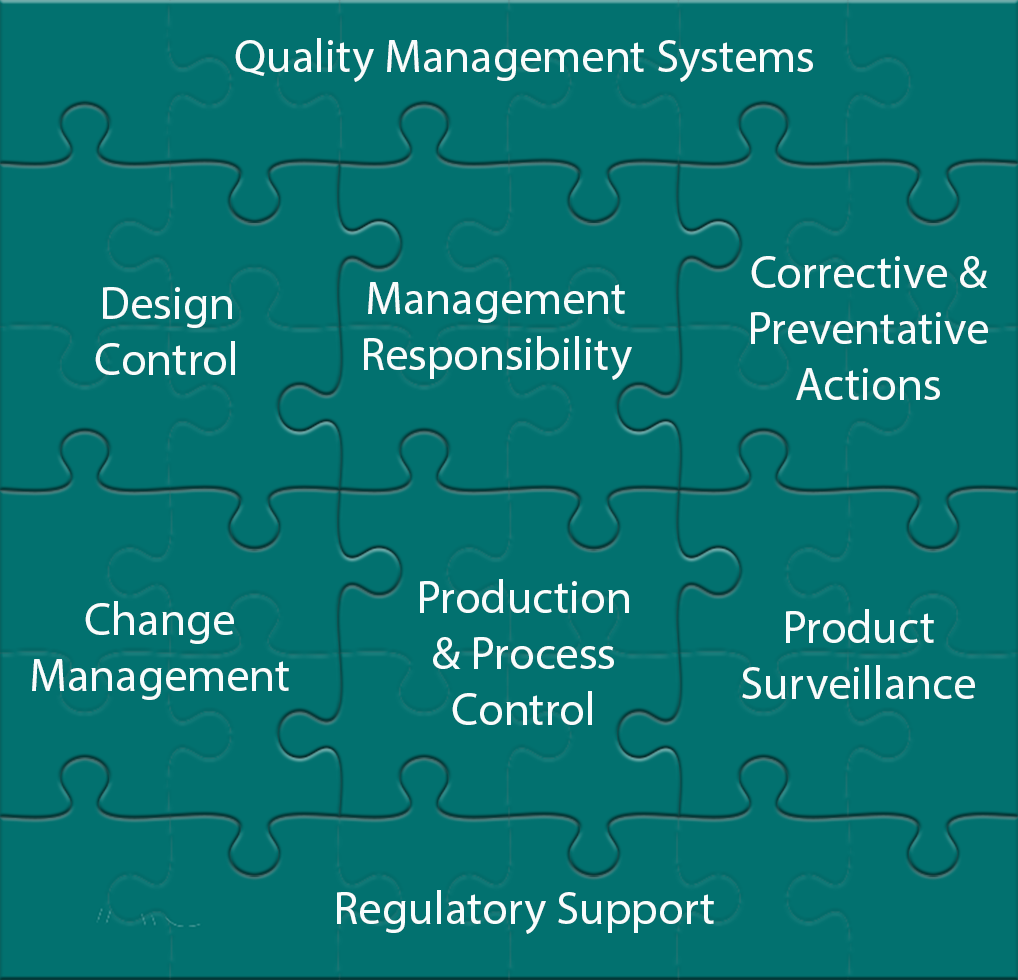
- 21 CFR 820 and ISO 13485 Compliant Quality Systems, Customized for your small business needs
- ISO 13485 Readiness Package (Preliminary review and mock audits to prepare your organization for the certification process)
- FDA Submission preparation (Establishment Registration, Device Listing, 510(k) preparation)
- FDA US Agent and/or Official Correspondent for foreign establishments
- Design Controls maintenance and consulting
- Regulatory strategy consulting

